The recent approval by the U.S. Food and Drug Administration (FDA) of the first nasal spray for the treatment of severe allergic reactions marks a significant advancement in emergency medicine and patient care. This innovative formulation, designed to address anaphylactic reactions—often triggered by allergens such as food, insect stings, or medications—provides an alternative route of administration that may enhance accessibility and ease of use during critical moments. Anaphylaxis is a life-threatening condition that demands immediate intervention, typically administered through epinephrine auto-injectors; however, these devices can be cumbersome and require specific training for effective usage.
The introduction of a nasal spray offers several potential advantages over traditional methods. Firstly, it eliminates the need for injections, which can be intimidating for certain populations, particularly children or individuals with needle phobia. Secondly, the nasal route allows for rapid absorption into systemic circulation, thereby facilitating quicker therapeutic effects compared to oral medications. This innovation could significantly reduce the time taken to manage acute allergic responses in emergency situations where every second counts.
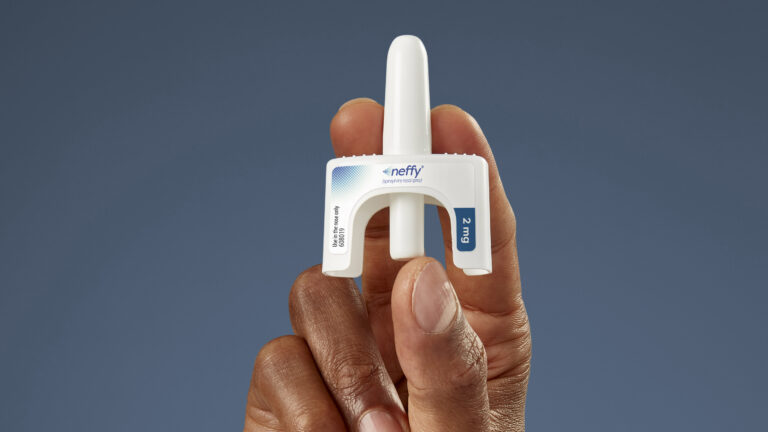
Neffy, as the product is called, is a nasal spray that delivers the same drug used to treat anaphylaxis. It is ARS’ flagship product. Friday’s approval comes after the FDA stalled neffy’s approval in September, asking the drugmaker for additional data.
Availability of a needle-free epinephrine option—comparable in price to injectables—may reduce barriers to rapid treatment of anaphylaxis, especially for those people who have a fear of injections.
Neffy’s approval was based on four studies that included 175 healthy adults without anaphylaxis. Concentrations of epinephrine in their blood after taking Neffy were comparable to those after using an auto-injector pen, according to the FDA.
Anaphylaxis is considered a medical emergency that patients should immediately seek medical care for. The severe, life-threatening reaction happens when a person's immune system reacts abnormally to a substance that normally does not cause symptoms. Some common allergens that could cause anaphylaxis are certain foods, medications and insect stings.
Symptoms can occur within minutes of exposure and include, but are not limited to, hives, swelling, itching, vomiting, difficulty breathing and loss of consciousness.
"Epinephrine is the only life-saving treatment for anaphylaxis and has previously only been available for patients as an injection," the FDA stated.
Access issues widen health disparities. Already, data suggest there are significant differences in food allergy prevalence by socioeconomic status. And Black, Hispanic, and Asian children have higher rates of severe allergic reactions, allergies to multiple foods, and related emergency visits, Ruchi Gupta, a pediatrician and founding director of the Center for Food Allergy & Asthma Research at Northwestern University’s Feinberg School of Medicine and Lurie Children’s Hospital of Chicago found in her research. (The top nine allergens are peanuts, tree nuts, shellfish, finned fish, milk, eggs, soy, wheat, and sesame.)
ARS will next look to expand the patient pool to children of lower weights. The drugmaker also told STAT it was conducting trials to see if neffy can work for people with chronic hives or persistent asthma.
Read more
Ukraine asserts control over vast areas of Russian territory, prompting tens of thousands to flee Trump-Musk conversation marred by tech issues, Musk tries to helpSarah H
Also on site :
- Gemini converts Google Docs to podcasts
- Khloé Kardashian responds to Lamar Odom’s blow-up sex doll of her: ‘So demonic and unwell’
- Jonathan Ross opens up about why he decided to quit alcohol