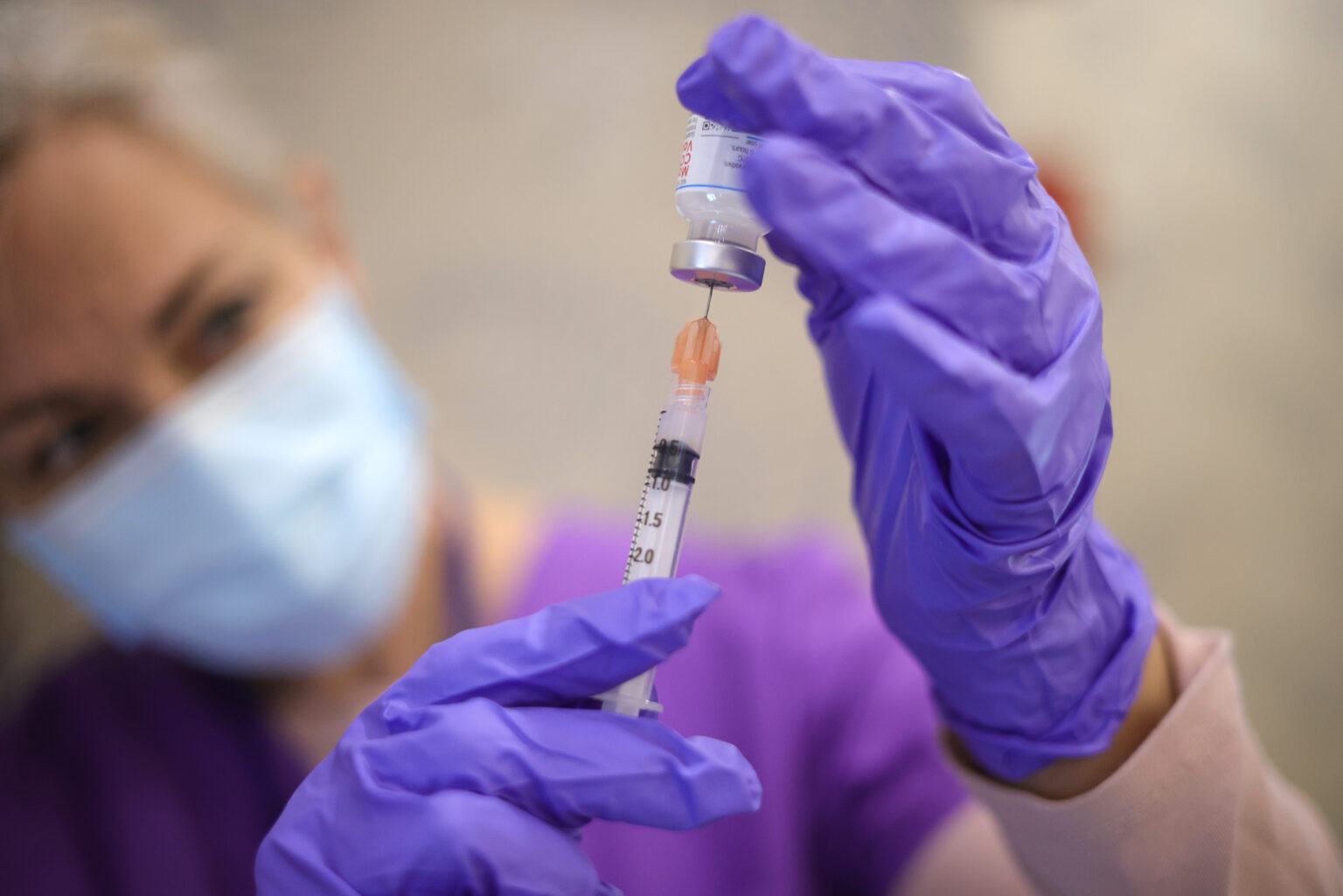
The recent announcement regarding the FDA's potential approval of updated COVID-19 vaccines marks a significant milestone in the ongoing battle against the pandemic. With the emergence of new variants, particularly those exhibiting increased transmissibility and immune evasion, public health officials have emphasized the necessity for vaccines that can effectively address these evolving challenges. The anticipated greenlight from the FDA is expected to facilitate a more robust immune response among populations previously vaccinated with earlier formulations, thereby enhancing community immunity and reducing hospitalizations.
Moreover, this development underscores the importance of adaptive strategies in vaccine formulation. Since the onset of COVID-19, scientists have continuously monitored viral mutations to ensure that vaccines remain effective. The forthcoming updated vaccines are designed to target specific variants that have become prevalent in recent months. This proactive approach not only reflects advancements in vaccine technology but also highlights a commitment to public health preparedness as we navigate through an era where viruses may continue to mutate.
In June, the US Centers for Disease Control and Prevention recommended that everyone over 6 months old receive both an updated Covid-19 vaccine and a flu shot this year.
Representatives for Pfizer and Moderna told CNN that the companies had ample supply of their updated Covid vaccines and would be ready to ship doses upon approval. Moderna’s spokesman said it expects the vaccine to be available in stores within days of FDA signoff.
Novavax’s vaccine is based on protein technology, which takes longer to manufacture than mRNA vaccines. The company’s executives told investors on a conference call last week that it anticipated that its updated vaccine would be arriving in warehouses this month and that it’s expected to be ready for distribution when authorized. A spokesperson for Novavax didn’t immediately respond to a request for comment Friday.
The complications raised by summer covid waves illustrate the downsides of releasing updated coronavirus vaccines alongside annual flu shots. Because demand for coronavirus vaccines has become low, federal officials regard that approach as a practical way to boost coverage and ensure Americans are protected by setting a predictable and familiar schedule, while making a second dose available in the spring for older and immunocompromised Americans.
But the decision to update the vaccine for the fall leaves Americans with weakened defenses against a virus that has shifted into new forms by summer. The existing vaccine targets XBB variants no longer in circulation and is not as effective against the latest variants, lab studies show. Experts say the older vaccines should still protect people against severe disease and hospitalization.
In addition to its scientific implications, the FDA's decision will likely impact public perception and willingness to receive booster shots. Clear communication regarding the safety and efficacy of these updated vaccines will be crucial in addressing vaccine hesitancy. As communities prepare for potential surges during colder months, fostering trust in vaccination programs will be essential for achieving widespread uptake and ultimately controlling transmission rates.
Read more
UFC Paris fight: Brendan Allen puts Nassourdine Imavov on blast Bregman, Pena homer as AL West-leading Astros win over RaysSarah H
Also on site :
- The Kardashians’ luxurious Easter baskets include a $900 designer purse
- The Last of Us’ Abby voice actor says HBO was wise to change controversial death after backlash
- The simple morning habits you should do to start your day, according to experts